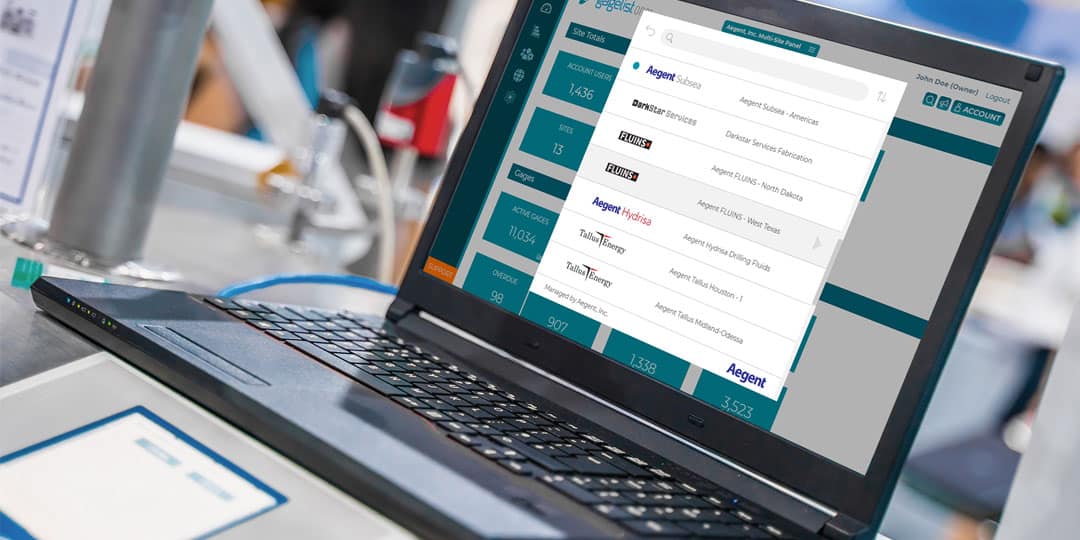
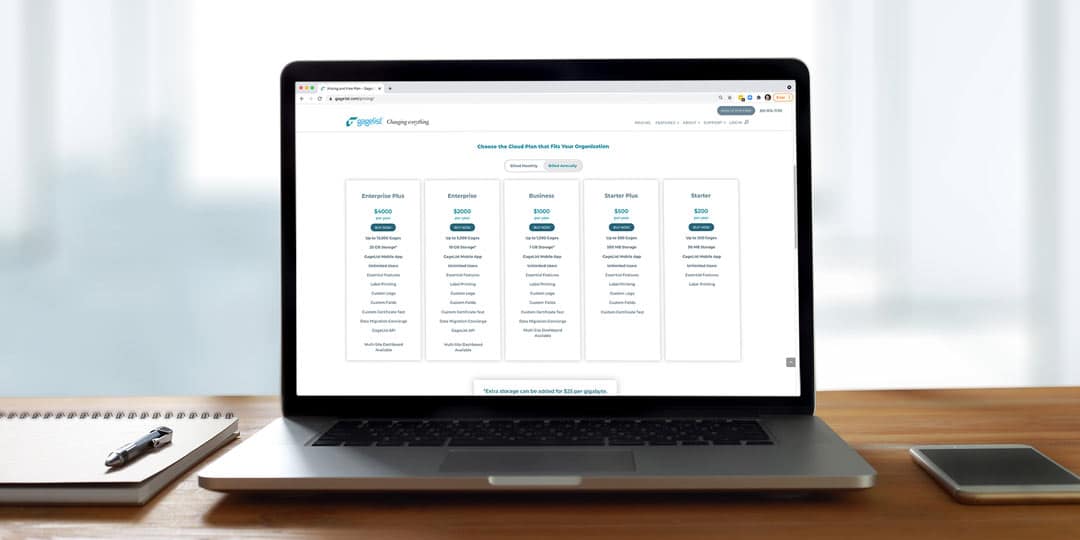
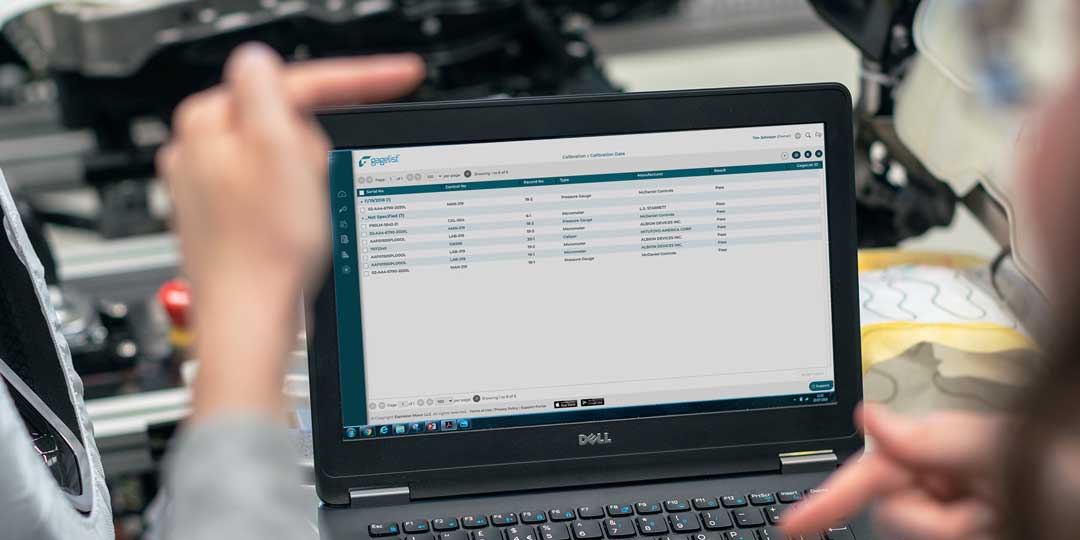
Calibration is a critical aspect of quality control for many industries, including manufacturing, aerospace, healthcare and more. For large enterprise companies with multiple locations, managing a calibration program can be a complex and time-consuming effort. This is...