GageList Blog
The Voice of the Calibration Revolution
Explore by Topic
Or View Most Recent

GageList Achieves SOC 2 Compliance
We are proud to announce that GageList, your trusted calibration management software provider, has achieved Service Organization Control (SOC) 2 compliance. SOC 2 is a robust framework designed by the American Institute of CPAs (AICPA), which is recognized as a gold...

Software Seat Licenses Are Dead. Long Live Unlimited Users!
Change management is about helping individuals, teams, and organizations transition from a current state to a desired future state. For many of us, that “future state” is rushing at us like a runaway train. Here's a look at how you're better prepared to manage change...

Six Reasons to Upgrade from Excel to GageList
If you haven't yet, it's time to upgrade from Excel to GageList. Calibrating measuring instruments generates a ton of data that has to be managed, not only to maintain compliance with your industry standards, but to ensure the products you deliver perform as expected....

Calibration of a New Instrument: Why It Matters
When a new measuring instrument arrives at your facility, you need to make sure it's properly calibrated before you put it into use. Calibration ensures that your gages produce accurate measurements; if they don't, you can't be confident that the goods you produce...

What is Calibration?
Calibration is the process of evaluating and adjusting a measuring instrument or system to ensure that it performs accurately and meets the desired specifications. This involves comparing the readings of the instrument to a known standard and making any necessary...
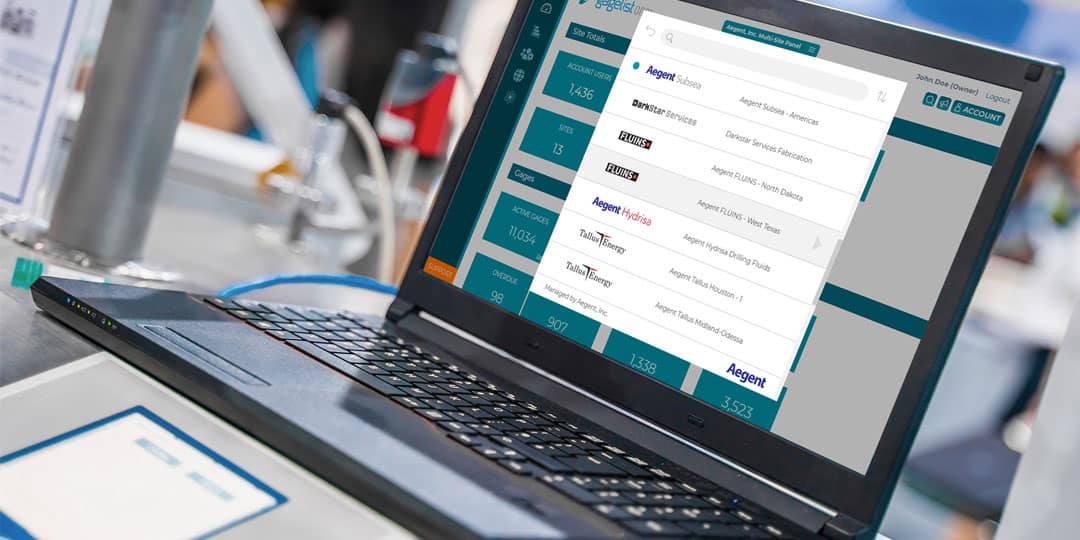
GageList Multi-Site Dashboard Delivers Unified Enterprise-Wide Calibration Management
Calibration is a critical aspect of quality control for many industries, including manufacturing, aerospace, healthcare and more. For large enterprise companies with multiple locations, managing a calibration program can be a complex and time-consuming effort. This is...

Digital Signatures for FDA Compliance
GageList offers true digital signatures for FDA compliance to support the requirements of FDA’s 21 CFR Parts 11 and 820, Quality System Regulation (QSR) and ISO 13485. The FDA requires organizations to employ means of capturing electronic signatures on electronic...
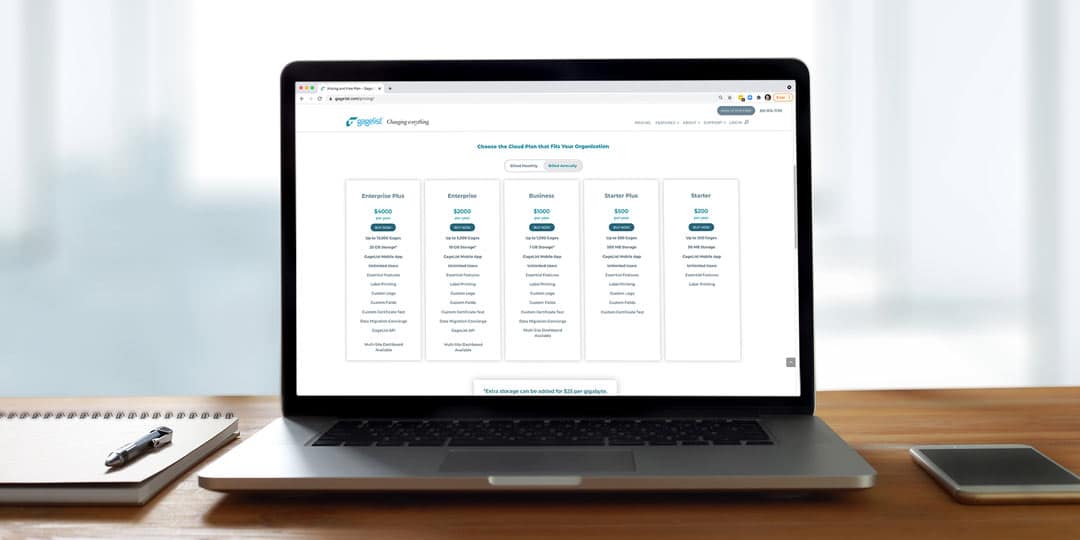
When Calibration Software Providers Won’t Show You Their Pricing Plans
Trying to find out the price of some calibration platforms is like trying to find that sock that disappeared when you did laundry last week. You look all over their website, you google it, you ask around, but you still can’t find it. Why is that? Could it be because...
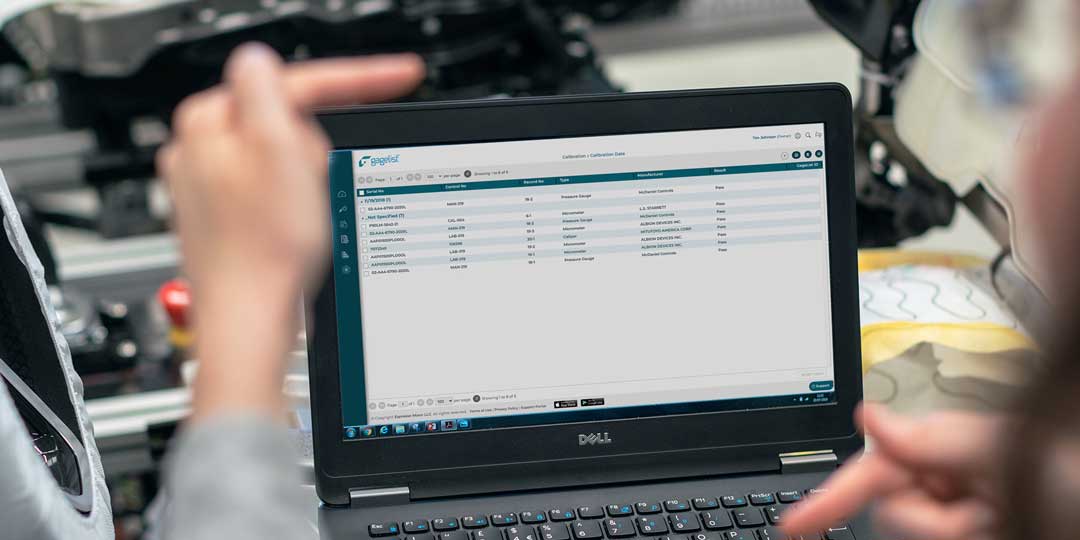
Custom Fields and Detailed Activity Log Provide Total Clarity
Every good calibration management software has a complex database with fields to accommodate the various types of data that an extensive gage list generates as calibrations and maintenance events occur. One measure of whether your calibration software is right for...

How Calibration Impacts Your Culture of Quality
Quality has never mattered more than it does today. Brilliant manufacturing technologies are raising the bar on what can be accomplished, and customers have access to information about product quality they’ve never had before. Advances in measurement technology are...